Arvida, always at the heart of innovation
The production of aluminum involves two main processes: the transformation of the raw material (called “bauxite”) into alumina, and the electrolysis of this alumina, which extracts the aluminum.
The refining plant is dedicated to the transformation of bauxite into alumina, which is filtered to remove ferrous impurities. During the dry process, the bauxite is dried, grinded, mixed with coke and heated to high temperature; the alumina is collected by precipitation. This processing method is suitable for bauxites of lower purity, such as anorthosite, which is found in the region's soil. In 1935, The Bayer process replaced the dry process that had been experimented with in the early years in Arvida; the bauxite to be refined is now imported from Guyana. The Bayer process consists of mixing bauxite with caustic soda in a high-pressure high-temperature pressure vessel. The result obtained is then separated from impurities by filtration and precipitation, then decomposed, washed and calcined. At the end of the refining process a white powder is obtained: this is alumina.
The second step is also energy-intensive: the electrolysis of alumina. The alumina is immersed in a carbon-lined pot containing mainly fluorspar (fluorite) and cryolite. A high-voltage electric current (hence the need for a current rectifier station) passes from the anode, which is dipped in the alumina mixture, to the cathode, which is the carbon lining of the pot. The alumina is thus stripped of its oxygen molecules and the resulting aluminum settles at the bottom of the pot.
Anodes must be replaced periodically as they are consumed; about three quarters of a ton of anode is used for each ton of aluminum produced. These anodes are therefore manufactured on site. Coke is calcined, pulverized, mixed with molten pitch, pressed into molds and “baked” in electric furnaces; this is what we call “pre-baked anodes”. The Soderberg smelting technology uses “raw” anodes. More efficient, but also more polluting, it was introduced in Arvida in 1938.
In 2013, Rio Tinto Alcan inaugurated 38 pot rooms in Arvida for a new technology called “AP60” (for “Aluminium Pechiney 600,000 amps”). The availability of hydroelectricity in Arvida supports this new electrolysis process based on the use of very high intensity current. This process considerably accelerates production while drastically reducing pollutant emissions.
In 2018, Alcoa collaborated with Rio Tinto to build an experimental carbon-free aluminum smelter in Arvida, as part of the company's ongoing search for less polluting methods. The technology called "Elysis" uses inert anodes that eliminate the production of greenhouse gases during the electrolysis process. In 2023, Rio Tinto announced the construction of an Elysis potline in Arvida.


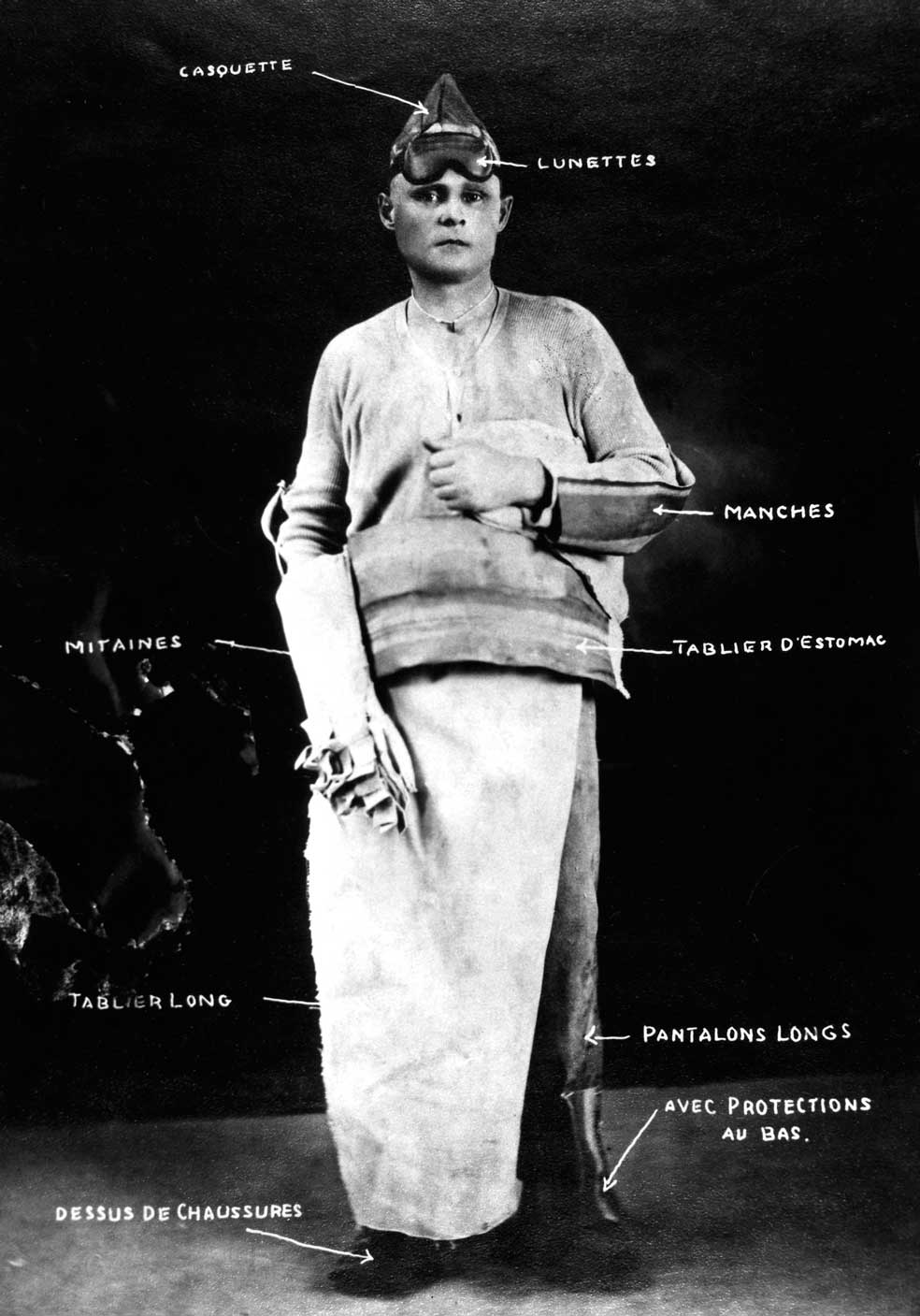



 Site officiel de la Ville de Saguenay
Site officiel de la Ville de Saguenay